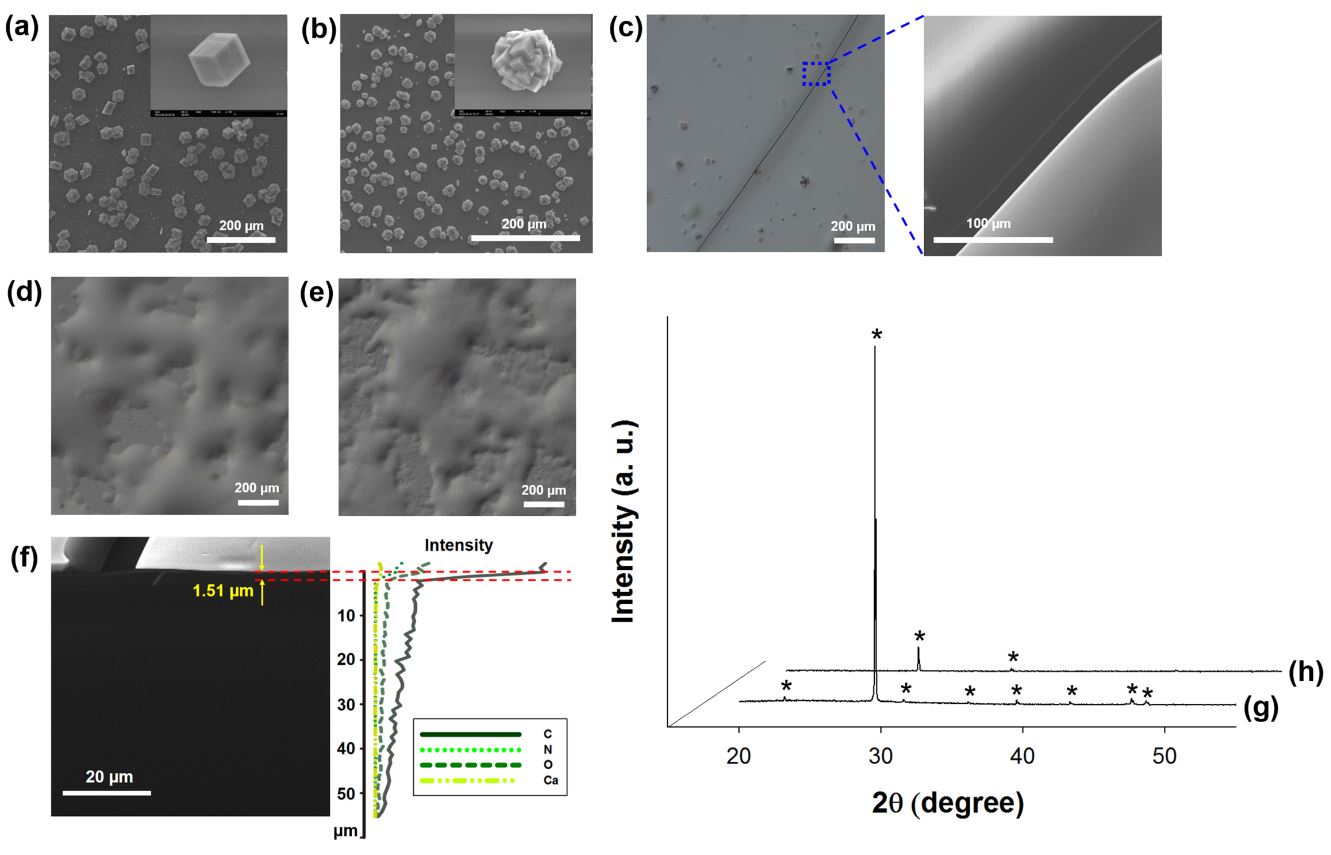
Coacervation is a liquid-liquid phase separation process of macromolecular polyelectrolytes. The formation of simple and complex coacervations of a synthetic acidic protein, GG1234, as a model shell matrix protein, was investigated with turbidity measurements and microscopic morphological observations. Simple coacervation of GG1234 was optimally induced at pH 3.75 and below 50 mM for all of the tested salts, and complex coacervates were prepared at pH 4–9 in sodium acetate solution at various ratios of GG1234 to lysozyme without inducing simple coacervation. The complex coacervates also had the ability to microencapsulate hydrophobic oil droplets in a similar manner to other complex coacervation systems. Remarkably, a thin film formed through in vitro CaCO3 crystallization in the presence of complex coacervates, which was expected to be planar and poorly crystalline CaCO3 guided at the interface of two immiscible liquid phases upon complex coacervation. Collectively, our results indicate that our synthetic acidic matrix protein could be used as the main protein for simple and complex coacervations, and the coacervates of this protein might be involved in thin film formation in CaCO3 crystallization.