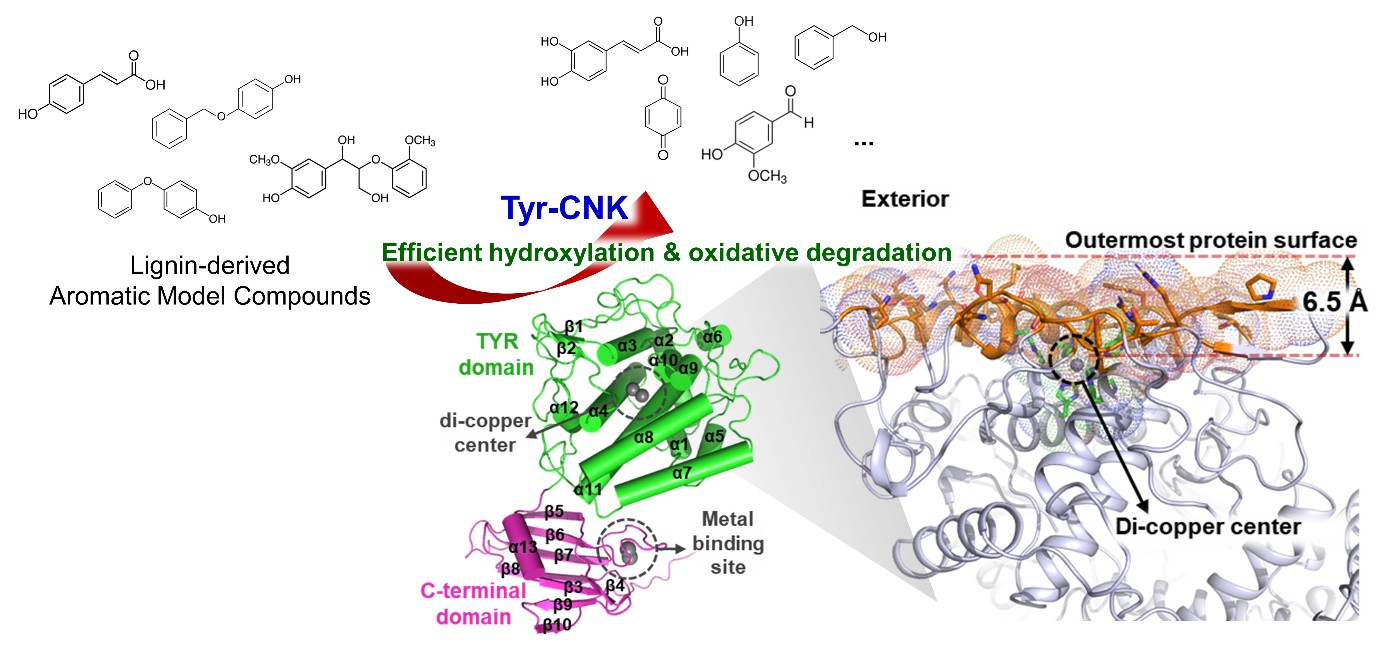
 Biomass-derived aromatic compounds, including those obtained from lignin (which is the most abundant aromatic biopolymer on Earth), are valuable for sustainable chemical production. Various lignin-degrading approaches have been developed to cleave recalcitrant bonds. The incorporation of biocatalysts that operate under environmentally friendly and mild conditions with high substrate specificity is considered one of the emerging strategies for lignin valorization. In this study, an archaeal tyrosinase (Tyr-CNK), derived from the marine archaeon Candidatus nitrosopumilus koreensis, is characterized as a versatile biocatalyst for lignin biodegradation and valorization, based on kinetic studies, protein structure determination, and analysis. Notably, the extremely shallow active site pocket and the unique noncanonical caddy domain, which facilitate efficient copper incorporation without obstructing the active site, collectively empower Tyr-CNK with remarkable catalytic efficiency toward various lignin model compounds, such as p-coumaric acid, 4-phenoxyphenol, 4-(benzyloxy)phenol, and guaiacyl glycerol-β-guaiacyl ether. Together with molecular docking simulations, these catalytic and structural features indicate that Tyr-CNK serves as an efficient biocatalyst for the hydroxylation and oxidative degradation of lignin-derived phenolic compounds. Given its versatility, efficiency, and structural uniqueness, Tyr-CNK demonstrates great promise for expanding the catalytic repertoire for biomass conversions and offering new opportunities in sustainable biocatalysis, enzymatic and microbial biodegradation and biomass valorization.
Biomass-derived aromatic compounds, including those obtained from lignin (which is the most abundant aromatic biopolymer on Earth), are valuable for sustainable chemical production. Various lignin-degrading approaches have been developed to cleave recalcitrant bonds. The incorporation of biocatalysts that operate under environmentally friendly and mild conditions with high substrate specificity is considered one of the emerging strategies for lignin valorization. In this study, an archaeal tyrosinase (Tyr-CNK), derived from the marine archaeon Candidatus nitrosopumilus koreensis, is characterized as a versatile biocatalyst for lignin biodegradation and valorization, based on kinetic studies, protein structure determination, and analysis. Notably, the extremely shallow active site pocket and the unique noncanonical caddy domain, which facilitate efficient copper incorporation without obstructing the active site, collectively empower Tyr-CNK with remarkable catalytic efficiency toward various lignin model compounds, such as p-coumaric acid, 4-phenoxyphenol, 4-(benzyloxy)phenol, and guaiacyl glycerol-β-guaiacyl ether. Together with molecular docking simulations, these catalytic and structural features indicate that Tyr-CNK serves as an efficient biocatalyst for the hydroxylation and oxidative degradation of lignin-derived phenolic compounds. Given its versatility, efficiency, and structural uniqueness, Tyr-CNK demonstrates great promise for expanding the catalytic repertoire for biomass conversions and offering new opportunities in sustainable biocatalysis, enzymatic and microbial biodegradation and biomass valorization.
아직 계정이 없으세요? 회원가입

69. E. Kang, Y. S. Choi, "Recent Advances on Enhancing Mass Transfer Efficiency in C1-Gas Bioconversion Processes", Korean Chem. Eng. Res. (2025) in press
Microbial bioconversion of C1-gases, such as methane and carbon monoxide, is a promising approach for sustainable carbon utilization but faces challenges due to poor gas solubility and mass-transfer li...

68. S.H. Lee, E. Kang, ... "Archaeal Tyrosinase as a Versatile Biocatalyst for Lignin-derived Aromatic Compounds Valorization", International Journal of Biological Macromolecules, (2025) 316:144669
Biomass-derived aromatic compounds, including those obtained from lignin (which is the most abundant aromatic biopolymer on Earth), are valuable for sustainable chemical production. Various lignin-degr...

67. D.Jeong, H. Choi, ...... "Coacervate formation of hBMP-2 fused with an ECM with HA", Biotechnology Bioprocess Eng. (2025) 30:455-463
Human bone morphogenetic protein-2 (hBMP-2) is a growth factor extensively used to promote bone regeneration. Nonetheless, challenges such as initial burst release, low solubility and aggregation under...

66. D.N. Kim, E. Kang, ...... "Molecular regime shift from kinetic limitation condition to mass-transfer limitation condition for stabilizing CO metabolism by microorganisms", Chemical Engineering Journal, 504:158801 (2025)
Carbon monoxide (CO) serves as a promising carbon source, but it adversely affects CO-metabolizing microorganisms. To address this issue, fermentation should occur under mass-transfer limitation condit...

65. G. Bae, J. Lee, H. Kim, Y.J. Yeon, Y.S. Choi, "Tyrosinase from Citreicella sp. as an organophilic enzyme for catechol biosynthesis", Biochemical Engineering Journal 201:109123 (2024)
Tyrosinases can directly catalyze the biosynthesis of catechol compounds without additional cofactors under mild conditions. However, the low stability of tyrosinases in organic media has limited their...

64. H. Choi, Y. Hong, S. Najafi, S.Y. Kim, J.-E. Shea, D.S. Hwang, Y.S. Choi, "Spontaneous transition of spherical coacervate to vesicle-like compartment", Advanced Science, 11:2305978 (2024)
Numerous biological systems contain vesicle-like biomolecular compartments without membranes, which contribute to diverse functions including gene regulation, stress response, signaling, and skin barri...

63. E. Moon, E. Kang, W. Song, B.J. Kim, H.J. Cha, Y.S. Choi, "Chitosan/oleamide electrospun nanofiber with enhanced spinnabiity and moderate hydrophobicity", KJChE 40:405-411 (2023)
Chitosan-based nanofibers have become attractive biomaterials for wound healing and dressing applications based on their intrinsic biocompatibility, biodegradability, and antibacterial properties. Howe...

62. E. Kang, E. Moon, W. Song, L.H. Kim, J.S. Hyung, J.-H. Jo, J.-H. Park, M.-S. Kim, J.-G. Na, Y.S. Choi, “Chitosan/oleamide nanofluid as a significant medium for enhancing gas utilization ......”, Chemical Engineering Journal, 433:133846 (2022)
Microbial biotransformation of C1 gas feedstocks such as CH4 and CO is a notable technique for sustainable, carbon–neutral chemical and fuel production. However, low C1-gas utilization efficiency in bi...

61. S. Kim, G. Bae, M. Shin, E. Kang, T.Y. Park, Y.S. Choi, H.J. Cha, “Oriented in situ immobilization of a functional tyrosinase on microcrystalline cellulose ......”, Biotechnololy Journal, 16:2100216, (2021)
Background: Catechol-containing polymers such as mussel adhesive proteins (MAPs) are attractive as biocompatible adhesive biomaterials, and the catecholic amino acid 3,4-dihydroxyphenyl-L-alanine (DOPA...

60. E. Kang, H.H. Je, E. Moon, J.-G. Na, M.S. Kim, D.S. Hwang, Y. S. Choi, “Cellulose nanocrystals coated with a tannic acid-Fe3+ complex as a significant medium for efficient CH4 microbial biotransformation”, Carbohydrate Polymers 258:117733, (2021)
Microbial biotransformation of CH4 gas has been attractive for the production of energy and high-value chemicals. However, insufficient supply of CH4 in a culture medium needs to be overcome for the ef...

59. Sujin Lim, Dawoon Jeong, Mi-Ran Ki, Seung Pil Pack, and Yoo Seong Choi, “Tyrosinase-mediated rapid and permanent chitosan/gelatin and chitosan/gelatin/nanohydroxyapatite hydrogel”, Korean J. Chem. Eng. 38:98-103 (2021)
Chitosan/gelatin and chitosan/gelatin/nanohydroxyapatite hydrogels were rapidly and stably prepared without any crosslinking materials by using an engineered tyrosinase (mTyr-CNK) with high catalytic a...

58. Chaeyeon Son, Wooho Song, Hyunsuk Choi, Yoo Seong Choi, “In vitro CaCO3 crystallization at room temperature and atmospheric pressure using recombinant proteins GRP_BA and GG1234”, Korean Chem. Eng. Res. 57:205-209, (2019)
The exquisite structure and attractive biological properties of biominerals have great potential and increased interest for use in a wide range of medical and industrial applications. Calcium carbonate...

57. Eun Hae Kim, Sujin Lim, Tae Eun Kim, In Oh Jeon, Yoo Seong Choi "Preparation of in situ injectable chitosan/gelatin hydrogel using an acid-tolerant tyrosinase," Biotechnology and Bioprocess Engineering 23:500-506 (2018)
An in situ injectable chitosan/gelatin hydrogel was formed under slightly acidic conditions (pH 4.0 ~ 4.5) using an acid-tolerant tyrosinase, tyrosinase-CNK. A homogeneous chitosan/tyrosinase-CNK solut...

56. Yun Jung Yang, Yoo Seong Choi, Hyung Joon Cha "Bioinspired load-bearing hydrogel based on engineered sea anemone skin-derived collagen-like protein", Biotechnology Journal 1800086 (2018)
With the help of recombinant DNA technology, many protein candidates have been investigated and engineered for biomaterial applications. Particularly, several repeat sequences with unique secondary str...

55. Eungsu Kang, Sun Young Kim, Yoo Seong Choi "Structural and functional properties of tyrosinases as a type-3 copper enzyme", KSBB Journal, 33:63-69 (2018)
Tyrosinases, as a representative type-3 copper enzyme (two copper ions in the active site), catalyze the orthohydroxylation of monophenols and the oxidation of diphenols. It is ubiquitously distributed...

54. Yoo Rae Choi, Eun Hae Kim, Sujin Lim, Yoo Seong Choi "Efficient preparation of a permanent chitosan/gelatin hydrogel using an acid-tolerant tyrosinase", Biochemical Engineering Journal, 129:50-56 (2018).
An acid-tolerant tyrosinase, tyrosinase-CNK, was used to form chitosan/gelatin hydrogels without any additional crosslinking materials at pH 4, where appropriate chitosan/gelatin blends were efficientl...

53. Hyunsu Do, Eungsu Kang, Byeongseon Yang, Hyung Joon Cha, Yoo Seong Choi "A tyrosinase, mTyr-CNK, that is functionally available as a monophenol monooxygenase", Scientific Reports, 7:17267 (2017).
Tyrosinase efficiently catalyzes the ortho-hydroxylation of monophenols and the oxidation of diphenols without any additional cofactors. Although it is of significant interest for the biosynthesis of c...

52. So Yeong Bahn, Byung Hoon Jo, Yoo Seong Choi, Hyung Joon Cha "Control of nacre biomineralization by Pif80 in pearl oyster", Science Advances, 3:e1700765 (2017).
Molluscan nacre is a fascinating biomineral consisting of a highly organized calcium carbonate composite that provides unique fracture toughness and an iridescent color. Organisms elaborately control t...

51. Chaeyeon Son, Sun Young Kim, So Yeong Bahn, Hyung Joon Cha, Yoo Seong Choi "CaCO3 thin-film formation mediated by a synthetic protein-lysozyme coacervate", RSC Advances, 7:15302-15308 (2017).
Coacervation is a liquid-liquid phase separation process of macromolecular polyelectrolytes. The formation of simple and complex coacervations of a synthetic acidic protein, GG1234, as a model shell ma...
50. Paulos Getachew, Mehader Getachew, Jin Joo, Yoo Seong Choi, Dong Soo Hwang, Yong-Ki Hong "The slip agents oleamide and erucamide reduce biofouling by marine benthic organisms (diatoms, biofilms and abalones)", ToxEHS, 8(5):341-348 (2016)
Primary fatty acid amides are commonly found in grasses, microalgae, and animal. Oleamide and erucamide are fatty acid amide derivatives of oleic and brassidic acids, respectively. They are the most fr...
Copyright 2017. Biomolecular Engineering Lab. All Right Reserved.