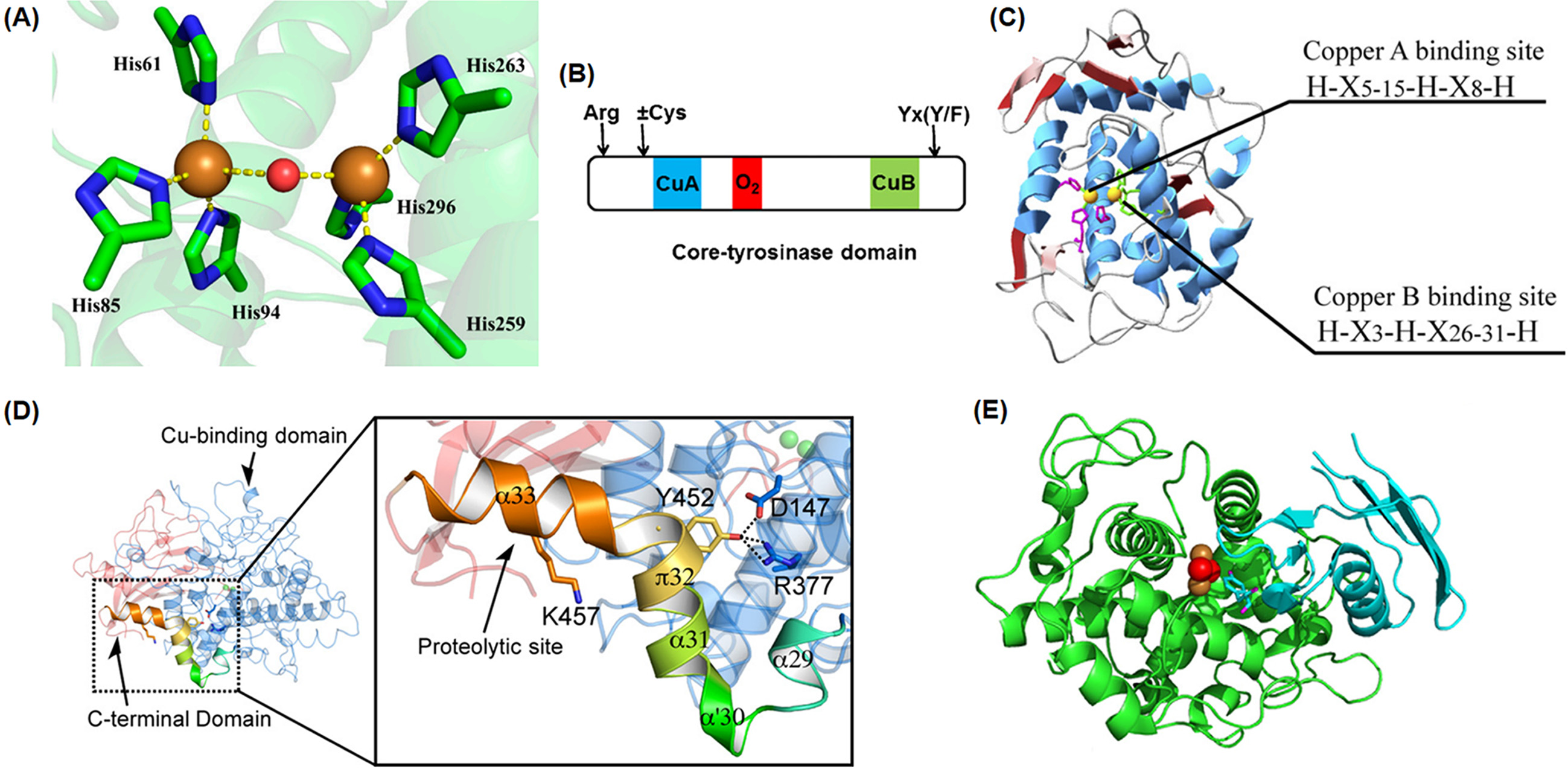
Tyrosinases, as a representative type-3 copper enzyme (two copper ions in the active site), catalyze the orthohydroxylation of monophenols and the oxidation of diphenols. It is ubiquitously distributed in various organisms all around the nature world. The catalytic mechanism of tyrosinases was intensively studied for a long time. On the other hand, some structural data have been reported recently. This review summarizes the structural information of overall threedimensional structure and active site region. The overall structure can be divided into three domains and some characteristic conserved sequences are observed, although tyrosinases do not have a common structure in all species. The catalytic mechanism is based on the structural characteristics that are significantly important to understand the functional properties in tyrosinases, which can be efficiently applied into industrial and biomedical applications of the enzymes.


















