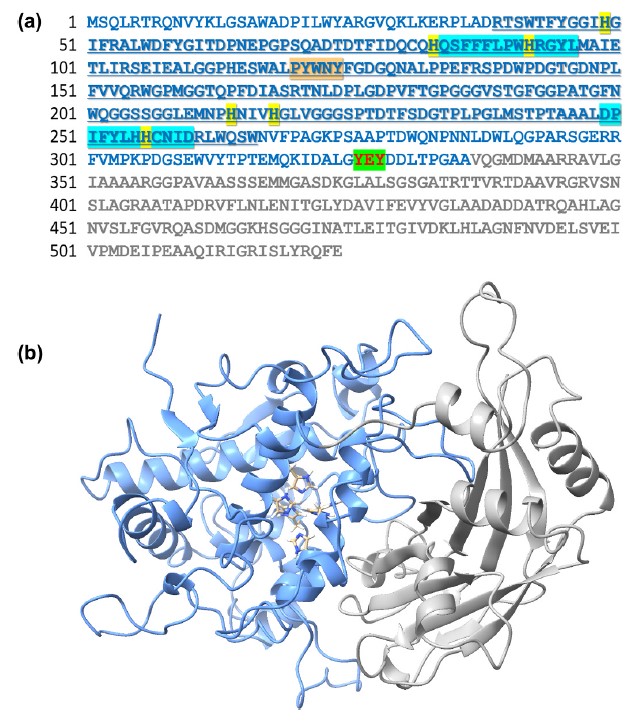
Tyrosinases can directly catalyze the biosynthesis of catechol compounds without additional cofactors under mild conditions. However, the low stability of tyrosinases in organic media has limited their use. Herein, a tyrosinase (csTyr) was identified from Citreicella sp. SE45 that possesses a characteristic feature of halophilic proteins and can be functionally expressed in Escherichia coli. Remarkably, the enzymatic activity of csTyr was effectively induced by primary alcohols in cosolvent media, whereas csTyr did not have enzymatic activity in aqueous media, minimizing unwanted reactions during tyrosinase preparation. In particular, the catalytic efficiency and stability of csTyr in a 50 % (v/v) ethanol-water cosolvent were comparable to those of other tyrosinases in water. Using csTyr as a catalyst for ortho-hydroxylation reactions of acetaminophen and resveratrol, the high catalytic activity and stability in 50 % (v/v) ethanol efficiently enabled the production of 3-hydroxy-acetaminophen and piceatannol. Therefore, csTyr, an organophilic tyrosinase, can be greatly useful in the biosynthesis of catechol compounds by addressing the issue of poor enzymatic stability in organic media.


















